TMS stimulates neurons in the prefrontal cortex, a region of the brain that is subactive in people with depression. TMS received clearance from the FDA (Food and Drug Administration) in 2008 for the treatment of people with depression for whom the drugs were not effective.
It can also be an alternative for women with depression who are pregnant or breastfeeding and therefore can choose not to take antidepressants. Unlike electro-convulsive therapy, TMS is not susceptible to causing seizures or memory loss. Insurance can cover TMS therapy.
TMS and tDCS
Repetitive Transcranial Magnetic Stimulation

TMS
Transcranial Magnetic Stimulation (TMS) uses a magnetic field generated by a coil in a shovel that is held against the patient's head to stimulate specific areas of the brain. This form of therapy is also called repetitive TMS or rTMS because the magnet is turned on and off quickly, creating an effect that feels as if someone is hitting your head.
Since 2012, a transcranial magnetic stimulation technique has had the Federal Council of Medicine (CFM), therefore, it is a medical act recognized as valid and used in the practice of medicine in Brazil.
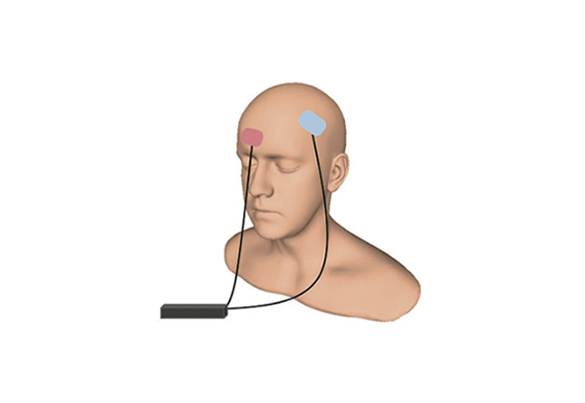
tDCS
Transcranial direct current stimulation (tDCS) transmits a weak current from a 9-volt battery (the size used in a smoke detector) through electrodes on the forehead or scalp. People who suffer from tDCS can feel their scalp tingle and hear a tinnitus. Each session lasts about 20 minutes.
The goal is to generate changes in brain excitability, that is, this technique is able to increase or reduce brain activity according to the location and positioning of the electrodes in the head.